What is a Mass Spectrometer?
A mass spectrometer is an instrument that measures the masses of indiviual molecules that
have been converted into ions, i.e., molecules that have been electrically
charged. Since molecules are so small, it is not convenient to measure their
masses is kilograms, or grams, or pounds. In fact, the mass of a single
hydrogen atom is approximately 1.66 X 10-24 grams. We therefore
need a more convenient unit for the mass of individual molecules. This unit of mass is often
referred to by chemists and biochemists as the Dalton (Da for short), and
is defined as follows: 1 Da=(1/12) of the mass of a single atom of the isotope
of carbon-12(12C). This follows the accepted convention of defining the 12C isotope
as having exactly 12 mass units. (Note that a resent suggestion is to call this unit a Thompson (Th).)
As will become clear in what follows, a mass spectrometer does not actually measure
the molecular mass directly, but rather the mass-to-charge ratio of the ions formed from the molecules. For
reasons similar to those discussed in the context of mass, it is inconvenient to measure
the charge on an individual ion in units appropriate to the macroscopic everyday world. A useful unit
for this purpose is the fundamental unit of charge, the magnitude of the charge on
an electron. It follows that the charge on an ion is denoted by the integer number z of
the fundamental unit of charge, and the mass-to-charge ratio m/z therefore represents Daltons per
fundamental unit of charge. In many cases, the ions encountered in mass spectrometry have just one charge (z=1) so the m/z value is
numerically equal to the molecular (ionic) mass in Da. Mass spectrometrists often speak loosely
of the "mass of an ion" when they really mean the m/z ratio, but this convenient way of speaking
is useful only for the case of singly-charged ions.
An actual mass spectrometer ranges in size from about the size of a home microwave oven
to large research instruments that dominate entire rooms. The different functional units of a mass
spectrometer are represented conceptually in the following block diagram (Figure 1).
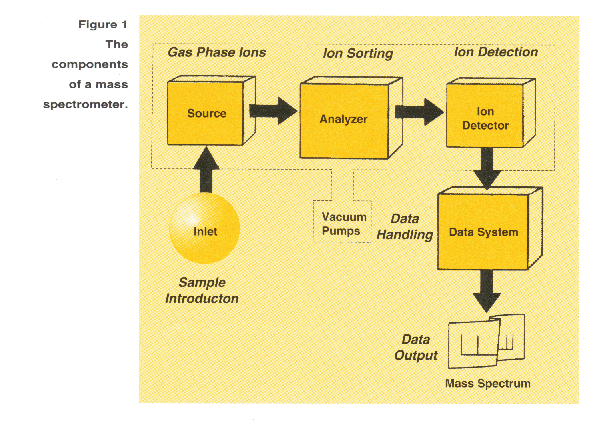
Formation of gas phase samples ions is an essential prerequisite to the
mass sorting and detection processes that occur in a mass spectrometer.
Early mass spectrometers required a sample to be a gas, but due to modern developments decribed below,
the applicability of mass spectrometry has been extended to include samples in
liquid solutions or embedded in a solid matrix. The sample, which may be a solid, liquid, or vapor, enters the vacuum chamber
through an inlet. Depending on the type of inlet and ionization techniques used, the sample may already
exist as ions in solution, or it may be ionized in conjunction with its volatilization or by other
methods in the ion source.
The gas phase ions are sorted in the mass analyzer according to their mass-to-charge (m/z) ratios and then collected
by a detector. In the detector the ion flux is coverted to a proportional electrical current. The data system records
the magnitude of these electrical signals as a function of m/z and converts this information into a mass spectrum.
Home
.gif)

.gif)

